|
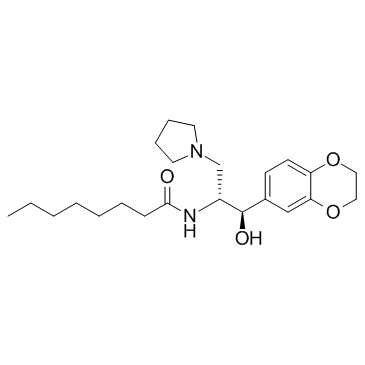
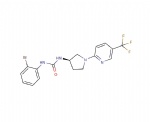
Product name : Eliglustat (Synonyms: Genz 99067)
Item : C2492
Price : 200mg, $459;500mg, $850; 1g, $1195; 2g, $1795
contact : Send inquiry to: info@acesobio.com
CAS : 491833-29-5 (free base)
Molecular Weight : 404.54
Formula : C₂₃H₃₆N₂O₄
Storage : at -20°C
Additional information : We offer significant discount for bulky quantity order.
 |
| Details: |
Description of:Eliglustat (Synonyms: Genz 99067)
Eliglustat(CAS:491833-29-5), also known as GENZ-112638, (trade name Cerdelga) is a treatment for Gaucher's disease developed by Genzyme Corp that was approved by the FDA August 2014. Commonly used as the tartrate salt, Eliglustat is believed to work by inhibition of glucosylceramide synthase. Quality control data: Quality control by 1H-NMR, 13C-NMR, HPLC and LCMS. Product will be shipped with supporting analytical data. REFERENCES1: Murugesan V, Chuang WL, Liu J, Lischuk A, Kacena K, Lin H, Pastores GM, Yang R, Keutzer J, Zhang K, Mistry PK. Glucosylsphingosine is a key biomarker of Gaucher disease. Am J Hematol. 2016 Nov;91(11):1082-1089. doi: 10.1002/ajh.24491. PubMed PMID: 27441734. 2: Pleat R, Cox TM, Burrow TA, Giraldo P, Goker-Alpan O, Rosenbloom BE, Croal LR, Underhill LH, Gaemers SJ, Peterschmitt MJ. Stability is maintained in adults with Gaucher disease type 1 switched from velaglucerase alfa to eliglustat or imiglucerase: A sub-analysis of the eliglustat ENCORE trial. Mol Genet Metab Rep. 2016 Sep 30;9:25-28. PubMed PMID: 27722092; PubMed Central PMCID: PMC5050260. 3: Belmatoug N, Di Rocco M, Fraga C, Giraldo P, Hughes D, Lukina E, Maison-Blanche P, Merkel M, Niederau C, Plӧckinger U, Richter J, Stulnig TM, Vom Dahl S, Cox TM. Management and monitoring recommendations for the use of eliglustat in adults with type 1 Gaucher disease in Europe. Eur J Intern Med. 2016 Aug 10. pii: S0953-6205(16)30217-5. doi: 10.1016/j.ejim.2016.07.011. [Epub ahead of print] Review. PubMed PMID: 27522145. 4: Cohen IJ, Baris H, Mistry PK, Sands MS. Overcoming the Next Barriers to Successful Therapy. Pediatr Endocrinol Rev. 2016 Jun;13 Suppl 1:629. PubMed PMID: 27491209. 5: Erratum: Profile of eliglustat tartrate in the management of Gaucher disease [Corrigendum]. Ther Clin Risk Manag. 2016 Jul 7;12:1083. doi: 10.2147/TCRM.S108027. PubMed PMID: 27462161; PubMed Central PMCID: PMC4939999. 6: Ibrahim J, Underhill LH, Taylor JS, Angell J, Peterschmitt MJ. Clinical response to eliglustat in treatment-naïve patients with Gaucher disease type 1: Post-hoc comparison to imiglucerase-treated patients enrolled in the International Collaborative Gaucher Group Gaucher Registry. Mol Genet Metab Rep. 2016 Jun 27;8:17-9. doi: 10.1016/j.ymgmr.2016.06.003. PubMed PMID: 27408819; PubMed Central PMCID: PMC4927653. 7: Smid BE, Ferraz MJ, Verhoek M, Mirzaian M, Wisse P, Overkleeft HS, Hollak CE, Aerts JM. Biochemical response to substrate reduction therapy versus enzyme replacement therapy in Gaucher disease type 1 patients. Orphanet J Rare Dis. 2016 Mar 24;11:28. doi: 10.1186/s13023-016-0413-3. PubMed PMID: 27008851; PubMed Central PMCID: PMC4806476. 8: Shayman JA. Targeting Glycosphingolipid Metabolism to Treat Kidney Disease. Nephron. 2016;134(1):37-42. doi: 10.1159/000444926. PubMed PMID: 26954668. 9: Sechi A, Dardis A, Bembi B. Profile of eliglustat tartrate in the management of Gaucher disease. Ther Clin Risk Manag. 2016 Jan 11;12:53-8. doi: 10.2147/TCRM.S73226. Review. Erratum in: Ther Clin Risk Manag. 2016;12:1083. PubMed PMID: 26811686; PubMed Central PMCID: PMC4714736. 10: Shayman JA. Developing novel chemical entities for the treatment of lysosomal storage disorders: an academic perspective. Am J Physiol Renal Physiol. 2015 Dec 15;309(12):F996-9. doi: 10.1152/ajprenal.00393.2015. Review. PubMed PMID: 26447223. 11: Yu J, Ritchie TK, Zhou Z, Ragueneau-Majlessi I. Key Findings from Preclinical and Clinical Drug Interaction Studies Presented in New Drug and Biological License Applications Approved by the Food and Drug Administration in 2014. Drug Metab Dispos. 2016 Jan;44(1):83-101. doi: 10.1124/dmd.115.066720. Review. PubMed PMID: 26424199. 12: Erratum: Eliglustat tartrate for the treatment of adults with type 1 Gaucher disease [Corrigendum]. Drug Des Devel Ther. 2015 Sep 11;9:5213. doi: 10.2147/DDDT.S95612. PubMed PMID: 26388688; PubMed Central PMCID: PMC4571929. 13: Balwani M, Burrow TA, Charrow J, Goker-Alpan O, Kaplan P, Kishnani PS, Mistry P, Ruskin J, Weinreb N. Recommendations for the use of eliglustat in the treatment of adults with Gaucher disease type 1 in the United States. Mol Genet Metab. 2016 Feb;117(2):95-103. doi: 10.1016/j.ymgme.2015.09.002. Review. PubMed PMID: 26387627. 14: Scott LJ. Eliglustat: A Review in Gaucher Disease Type 1. Drugs. 2015 Sep;75(14):1669-78. doi: 10.1007/s40265-015-0468-9. Review. PubMed PMID: 26384672. 15: Bennett LL, Turcotte K. Eliglustat tartrate for the treatment of adults with type 1 Gaucher disease. Drug Des Devel Ther. 2015 Aug 18;9:4639-47. doi: 10.2147/DDDT.S77760. Review. Erratum in: Drug Des Devel Ther. 2015;9:5213. PubMed PMID: 26345314; PubMed Central PMCID: PMC4554398. 16: Dagher R, Watzinger M, Chevalier G, Thirion-Delalande C, Gervais F, Forster R. Carcinogenicity testing of eliglustat in mice and rats. Regul Toxicol Pharmacol. 2015 Oct;73(1):401-12. doi: 10.1016/j.yrtph.2015.07.024. PubMed PMID: 26232705. 17: Grzegorek K. [New oral therapy option]. MMW Fortschr Med. 2015 Jul 23;157(13):77. doi: 10.1007/s15006-015-3362-1. German. PubMed PMID: 26206047. 18: Eliglustat (Cerdelga)--An Oral Drug for Gaucher Disease. Med Lett Drugs Ther. 2015 Jul 6;57(1472):e100-1. Review. PubMed PMID: 26147895. 19: Hughes DA, Pastores GM. Eliglustat for Gaucher's disease: trippingly on the tongue. Lancet. 2015 Jun 13;385(9985):2328-30. doi: 10.1016/S0140-6736(15)60206-9. PubMed PMID: 25819692. 20: Cox TM, Drelichman G, Cravo R, Balwani M, Burrow TA, Martins AM, Lukina E, Rosenbloom B, Ross L, Angell J, Puga AC. Eliglustat compared with imiglucerase in patients with Gaucher's disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015 Jun 13;385(9985):2355-62. doi: 10.1016/S0140-6736(14)61841-9. Erratum in: Lancet. 2015 Jun 13;385(9985):2354. PubMed PMID: 25819691. |
| Related Products : |
|
|
|
|
||||||||
|
|
|
|



 Apoptosis
Apoptosis
 MAO
MAO